Describe Solutions as Homogeneous Mixture and Its Uses
Homogeneous mixtures exist in one phase of matter at a time. Suspensions and colloids are also homogenous mixtures.

Solutions Unit 3 Solution It Is A Homogeneous Mixture That Is Formed When A Substance Is Dissolved In Another Substance Ppt Download
Solution - Definition A solution is a homogeneous mixture of two or more substances elements or compounds in no definite ratio by mass.

. If its composition is uniform throughout it is a homogeneous mixture. For example if I add a tablespoon of sugar to a cup of water and mix it you would see that all the sugar dissolves into the water and no longer can see the individual sugar cubes. You cant separate out the parts of natural gas.
Either a heterogeneous mixture or a homogeneous mixture. So a solution is a homogenous mixture. A homogeneous mixture has the same uniform appearance and composition throughout.
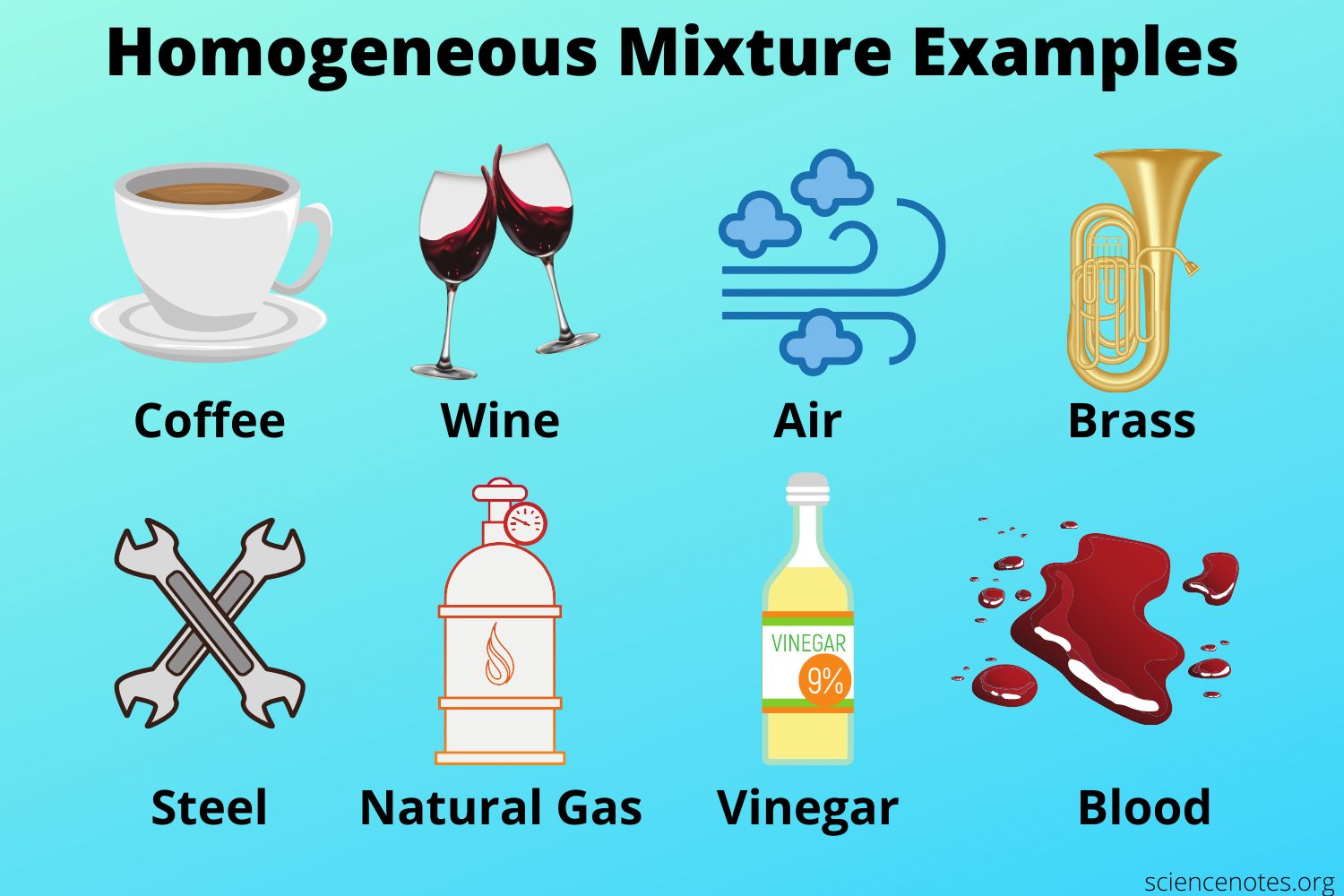
All solutions are mixtures because it is. It is uniform in composition throughout. Natural gas - a gaseous homogeneous mixture of methane and other hydrocarbons used as a fuel.
It is usually separated from tea leaves by filtration. Solution A Tea is a solution of compounds in water so it is not chemically pure. A homogeneous solution is a mixture of two or more components that have a uniform appearance and composition.
This mixture that we use for cooking is also homogeneous. If a substance is not chemically pure it is either a heterogeneous mixture or a homogeneous mixture. A stock or standard solution is a solution in which.
At first glance both ingredients can not be detected. It is usually separated from tea leaves by filtration. A solution is a special type of homogeneous mixture where the ratio of solute to solvent remains the same throughout the solution and the particles are not visible with the naked eye even if homogenized with multiple sources.
Evaporation is a technique used to separate out homogeneous mixtures that contain one or more dissolved salts. Seperation of Homogeneous Mixture. It has only one phase.
Solutions can exist in any phase Liquid solutions must be clear light can pass through and can be colored. Its components are not visible. The mixture is stable the particles do not settle at the down Its particle size is often at atomic or molecular level.
All homogeneous mixtures are solutions. Why can a mixture be separated by physical methods. The method drives off the liquid components from the solid components.
B Because the composition of the solution is uniform throughout it is a. Homogeneous mixtures that are thoroughly mixed down to the level of molecules are called solutions. Homogeneous solutions should not be confused with heterogeneous suspensions in which the components are made up of larger and less-uniform particles.
Weve got the study and writing resources you need for your assignments. Nitrous oxide - one of many gaseous homogeneous mixtures used for anesthesia. This mixture is composed of various gaseous substances such as carbon dioxide nitrogen oxygen and ozone among other gases.
Followings are the methods for separation of homogeneous mixture. As anesthesia nitrous oxide is used in a 5050 solution with oxygen. Homogeneous mixtures are known as solutions.
Homogeneous mixture a combination of two or more substances that have uniform composition and chemical properties throughout. You will not see liquid water and solid water together in a homogeneous mixture. Solution A Tea is a solution of compounds in water so it is not chemically pure.
A solution is a homogeneous mixture in which one or more substances the solutes are dissolved into another substance the solvent. A solution is a specific term that describes an even or homogeneous mixture of a solute the substance being mixed in a solvent the substance that is in a greater amount in which the solute dissolves. Atom molecule pure substance homogeneous mixture heterogeneous mixture element solution-Table salt sodium chloride or NaCl-Blue Cheese salad dressing-Iron Fe.
Academiaedu is a platform. Alloys are the examples of solution. In fact doctors colloquially refer to nitrous oxide as gas and air.
Many homogeneous mixtures are commonly referred to as solutions. When oil and water are mixed the resultant mixture is heterogeneous. Mixtures that are not homogeneous are said to be heterogeneous.
At first glance both ingredients can not be detected. Solutions Reading the warning labels that describe what happens when the chemical comes in contact with skin or eyes or is ingested will emphasize the need for chemical resistance in the plastic packaging. It cannot be seperated out physically.
If the mixture has one phase it is homogeneous. Solution is the general term used to describe homogenous mixtures with small particles. A homogeneous mixture is a mixture of two or more substances that are spread evenly and thoroughly throughout the entire solution.
First week only 499. A homogeneous mixture is a gaseous liquid or solid mixture that has the same proportions of its components throughout a given sample. Alcohol and water is homogeneous.
When salt and water are mixed they form a homogenous solution. Describe how homogeneous mixture differs from a heterogeneous mixture. B Because the composition of the solution is uniform throughout it is a homogeneous.
What is a solution. A solid substance is dissolved in a liquid substance is a solution. Homogenous means similar throughout or of a simlar kind though see below.
Flour with icing sugar. What is a Homogeneous Mixture. Steel is an example of solid in solid mixture.
Start your trial now. There is only one phase of matter observed in a homogeneous mixture. DAY 2 Describe solutions as homogeneous mixture and its uses.
If its composition is uniform throughout it is a homogeneous mixture. I NEED HELP PLEASE THANKS Describe each of the following compounds with as many of these words as apply. A heterogeneous mixture consists of visibly different substances or phases.
An example of a homogeneous mixture would be something like lemonade. The term homogeneous is used to describe portions of matter that are uniform throughout. A mixture is made up of two or more substances which are not chemically combined.
Muddy water and ice water are heterogenous mixtures while pure water is a compound and a pure substance. Solution for Describe how a homogeneous mixture differs from a heterogeneous mixture. What are homogeneous mixtures.
Raise YES if the mixture being described is homogeneous and not if its not. Carbonated water vodka and saline are all examples of homogeneous solutions. Examples of Homogeneous Mixture.
Todays lesson is a continuation of the first module that we have learned last time. Particle size distinguishes homogeneous solutions from other heterogeneous mixtures.

Classifications Of Mixtures Heterogeneous Mixtures Composed Of Different Types Of Phases Of Substances Ex Fruit Salad Granite Homogeneous Mixtures The Ppt Download
Is Sugar A Homogeneous Or Heterogeneous Mixture Quora

Solutions Types Of Mixtures Objectives 1 Distinguish Between Hetergeneous And Homogeneous Mixtures 2 List Different Solute Solvent Combinations 3 Compare Ppt Download

Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Combinat Chemical Science Solutions And Mixtures Materials Science

Homogeneous Mixture Definition Examples Tutors Com

Examples Of Homogeneous Mixtures Solid Liquid And Gas

Solutions Unit 3 Solution It Is A Homogeneous Mixture That Is Formed When A Substance Is Dissolved In Another Substance Ppt Download

What Is A Solution Components Characteristics Concentration Types

Homogeneous Mixture Definition Lesson For Kids Video Lesson Transcript Study Com

Homogeneous Mixture Definition Examples Tutors Com

Homogeneous Solution Overview Examples What Is A Homogeneous Mixture Video Lesson Transcript Study Com
What Are Homogeneous Mixtures Examples Techicy

10 Homogeneous Mixture Examples In Daily Life Studiousguy

Homogeneous Mixture Definition Examples Tutors Com

3 4 Classifying Matter According To Its Composition Chemistry Libretexts

Lesson Explainer Mixtures Nagwa
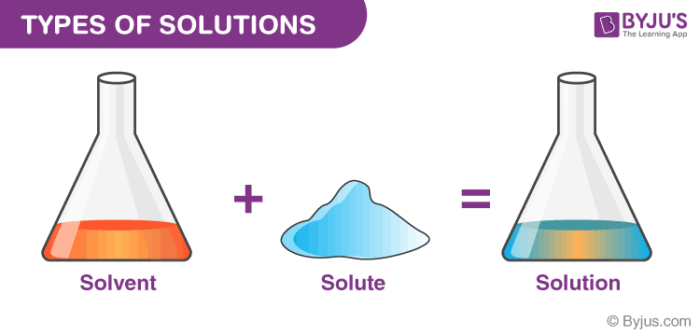
Types Of Solutions Different Types Homogeneous Heterogeneous Solution With Videos

Comments
Post a Comment